 |
| These are pictures of Daniel at seven and eight. |
Because Daniel was well and had no known cardiac issues, conduction or otherwise, no baseline EKG was ever obtained in his twelve and a half years on Earth. And so, when he experienced a sudden death with no reasons found on multiple autopsies afterward, the medical examiners assigned a presumptive cause of death. A baseline EKG could not be used to determine whether there were signs of bundle branch block, atrial fibrillation, or a "J-wave". Based on our family history of elderly members having conduction disorders, and the character of Daniel's sudden death, and inability to respond to immediate CPR, the cause of death is surmised to be a cardiac conduction disorder which evolved into a ventricular arrhythmia which was incompatible with life. There are a number of types of conduction disorders which can impact babies, children, teens and young adults. Today, Medscape sent me additional medical continuing education for my RN license. Although Brugada Syndrome does have some commonalities with Daniel's case, there are also great differences.
Normally, when I write an informational or educational post on this blog, I paraphrase the information on the most recent studies in order to be more readily understood by the general public. In this case however, I thought this latest article written by cardiologists was so important, that I should include it verbatim, crediting both Medscape and the Authors, Jose M. Dizon, MD, and Jeffrey N. Rottman, MD. If you or your family have anyone who experienced a sudden cardiac death, then the article which follows should probably be printed out and taken to your physician, so that other family members can be screened and potentially treated to avoid the potential for sudden arrhythmiccardiac death.
If this post saves even one life, then the article, and this post will have done its job.
_________________________
Brugada Syndrome
Author: Jose M Dizon, MD; Chief Editor: Jeffrey N Rottman, MDPractice Essentials
Brugada
syndrome is a disorder characterized by sudden death associated with one
of several ECG patterns characterized by incomplete right bundle-branch
block and ST-segment elevations in the anterior precordial leads.
See Clinical Presentation for more detail.
Testing
In patients with suspected Brugada syndrome, consider the following studies:
Imaging studies
Perform echocardiography and/or MRI, primarily to exclude arrhythmogenic right ventricular cardiomyopathy, as well as to assess for other potential causes of arrhythmias.
See Workup for more detail.
No pharmacologic therapy has been proven to reduce the occurrence of ventricular arrhythmias or sudden death; however, theoretically, drugs that counteract the ionic current imbalance in Brugada syndrome could be used to treat it. For example, quinidine, which blocks the calcium-independent transient outward potassium current (Ito), has been shown to normalize the ECG pattern in patients with Brugada syndrome.[4] However, quinidine also blocks sodium (Na) currents, which could have contrary effects.
See Treatment and Medication for more detail.
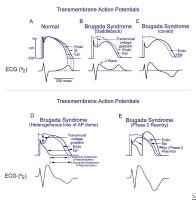 Schematics
show the 3 types of action potentials in the right ventricle:
endocardial (End), mid myocardial (M), and epicardial (Epi). A, Normal
situation on V2 ECG generated by transmural voltage gradients during the
depolarization and repolarization phases of the action potential. B-E,
Different alterations of the epicardial action potential that produce
the ECG changes observed in patients with Brugada syndrome. Adapted from
Antzelevitch, 2005.
Schematics
show the 3 types of action potentials in the right ventricle:
endocardial (End), mid myocardial (M), and epicardial (Epi). A, Normal
situation on V2 ECG generated by transmural voltage gradients during the
depolarization and repolarization phases of the action potential. B-E,
Different alterations of the epicardial action potential that produce
the ECG changes observed in patients with Brugada syndrome. Adapted from
Antzelevitch, 2005.
Essential update: Predicting prognosis in Brugada syndrome with J-wave characteristics
In patients with Brugada syndrome, the presence of a J wave in multiple leads and horizontal ST-segment morphology after J wave are associated with a higher incidence of cardiac events, according to a recent study of 460 patients with Brugada syndrome.[1] Important predictors of cardiac events included symptoms, QRS duration in lead V2 longer than 90 ms, and inferolateral J wave and/or horizontal ST-segment morphology after J wave.[1]Signs and symptoms
Signs and symptoms in patients with Brugada syndrome may include the following:- Syncope and cardiac arrest: Most common clinical manifestations; in many cases, cardiac arrest occurs during sleep or rest
- Nightmares or thrashing at night
- Asymptomatic, but routine ECG shows ST-segment elevation in leads V1-V3
- Associated atrial fibrillation (20%)[2]
- Fever: Often reported to trigger or exacerbate clinical manifestations
See Clinical Presentation for more detail.
Diagnosis
Most patients with Brugada syndrome have a normal physical examination. However, such an examination is necessary to exclude other potential cardiac causes of syncope or cardiac arrest in an otherwise healthy patient (eg, heart murmurs from hypertrophic cardiomyopathy or from a valvular or septal defect).Testing
In patients with suspected Brugada syndrome, consider the following studies:
- 12-lead ECG in all patients with syncope
- Drug challenge with a sodium channel blocker in patients with syncope without an obvious cause
- Electrophysiologic study to determine the inducibility of arrhythmias for risk stratification
- Serum potassium and calcium levels: In patients presenting with ST-segment elevation in the right precordial leads
- Potassium and calcium levels: ECG patterns in patients with hypercalcemia and hyperkalemia similar to that of Brugada syndrome
- CK-MB and troponin levels: In patients with symptoms compatible with an acute coronary syndrome
- Genetic testing for a mutation in SCN5A
Imaging studies
Perform echocardiography and/or MRI, primarily to exclude arrhythmogenic right ventricular cardiomyopathy, as well as to assess for other potential causes of arrhythmias.
See Workup for more detail.
Management
To date, the only treatment that has proven effective in treating ventricular tachycardia and fibrillation and preventing sudden death in patients with Brugada syndrome is implantation of an automatic implantable cardiac defibrillator (ICD).No pharmacologic therapy has been proven to reduce the occurrence of ventricular arrhythmias or sudden death; however, theoretically, drugs that counteract the ionic current imbalance in Brugada syndrome could be used to treat it. For example, quinidine, which blocks the calcium-independent transient outward potassium current (Ito), has been shown to normalize the ECG pattern in patients with Brugada syndrome.[4] However, quinidine also blocks sodium (Na) currents, which could have contrary effects.
See Treatment and Medication for more detail.
Image library
 Schematics
show the 3 types of action potentials in the right ventricle:
endocardial (End), mid myocardial (M), and epicardial (Epi). A, Normal
situation on V2 ECG generated by transmural voltage gradients during the
depolarization and repolarization phases of the action potential. B-E,
Different alterations of the epicardial action potential that produce
the ECG changes observed in patients with Brugada syndrome. Adapted from
Antzelevitch, 2005.
Schematics
show the 3 types of action potentials in the right ventricle:
endocardial (End), mid myocardial (M), and epicardial (Epi). A, Normal
situation on V2 ECG generated by transmural voltage gradients during the
depolarization and repolarization phases of the action potential. B-E,
Different alterations of the epicardial action potential that produce
the ECG changes observed in patients with Brugada syndrome. Adapted from
Antzelevitch, 2005. Background
Brugada syndrome
is a disorder characterized by sudden death associated with one of
several electrocardiographic (ECG) patterns characterized by incomplete
right bundle-branch block and ST elevations in the anterior precordial
leads. See the image below.
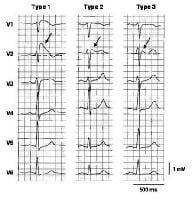 Three
types of ST-segment elevation in Brugada syndrome, as shown in the
precordial leads on ECG in the same patient at different times. Left
panel shows a type 1 ECG pattern with pronounced elevation of the J
point (arrow), a coved-type ST segment, and an inverted T wave in V1 and
V2. The middle panel illustrates a type 2 pattern with a saddleback
ST-segment elevated by >1 mm. The right panel shows a type 3 pattern
in which the ST segment is elevated < 1 mm. According to a consensus
report (Antzelevitch, 2005), the type 1 ECG pattern is diagnostic of
Brugada syndrome. Modified from Wilde, 2002. In the initial
description of Brugada syndrome, the heart was reported to be
structurally normal, but this concept has been challenged.[5] Subtle structural abnormalities in the right ventricular outflow tract have been reported.
Three
types of ST-segment elevation in Brugada syndrome, as shown in the
precordial leads on ECG in the same patient at different times. Left
panel shows a type 1 ECG pattern with pronounced elevation of the J
point (arrow), a coved-type ST segment, and an inverted T wave in V1 and
V2. The middle panel illustrates a type 2 pattern with a saddleback
ST-segment elevated by >1 mm. The right panel shows a type 3 pattern
in which the ST segment is elevated < 1 mm. According to a consensus
report (Antzelevitch, 2005), the type 1 ECG pattern is diagnostic of
Brugada syndrome. Modified from Wilde, 2002. In the initial
description of Brugada syndrome, the heart was reported to be
structurally normal, but this concept has been challenged.[5] Subtle structural abnormalities in the right ventricular outflow tract have been reported.
Brugada syndrome is genetically determined and has an autosomal dominant pattern of transmission in about 50% of familial cases (see Etiology). The typical patient with Brugada syndrome is young, male, and otherwise healthy, with normal general medical and cardiovascular physical examinations.
Patients with Brugada syndrome are prone to develop ventricular tachyarrhythmias that may lead to syncope, cardiac arrest, or sudden cardiac death.[6, 7, 8] Infrahisian conduction delay and atrial fibrillation may also be manifestations of the syndrome.[9, 10]
About 5% of survivors of cardiac arrest have no clinically identified cardiac abnormality. About half of these cases are thought to be due to Brugada syndrome.[11]
At present, implantation of an automatic implantable cardiac defibrillator (ICD) is the only treatment proven effective in treating ventricular tachycardia and fibrillation and preventing sudden death in patients with Brugada syndrome (see Treatment).
 Three
types of ST-segment elevation in Brugada syndrome, as shown in the
precordial leads on ECG in the same patient at different times. Left
panel shows a type 1 ECG pattern with pronounced elevation of the J
point (arrow), a coved-type ST segment, and an inverted T wave in V1 and
V2. The middle panel illustrates a type 2 pattern with a saddleback
ST-segment elevated by >1 mm. The right panel shows a type 3 pattern
in which the ST segment is elevated < 1 mm. According to a consensus
report (Antzelevitch, 2005), the type 1 ECG pattern is diagnostic of
Brugada syndrome. Modified from Wilde, 2002. In the initial
description of Brugada syndrome, the heart was reported to be
structurally normal, but this concept has been challenged.[5] Subtle structural abnormalities in the right ventricular outflow tract have been reported.
Three
types of ST-segment elevation in Brugada syndrome, as shown in the
precordial leads on ECG in the same patient at different times. Left
panel shows a type 1 ECG pattern with pronounced elevation of the J
point (arrow), a coved-type ST segment, and an inverted T wave in V1 and
V2. The middle panel illustrates a type 2 pattern with a saddleback
ST-segment elevated by >1 mm. The right panel shows a type 3 pattern
in which the ST segment is elevated < 1 mm. According to a consensus
report (Antzelevitch, 2005), the type 1 ECG pattern is diagnostic of
Brugada syndrome. Modified from Wilde, 2002. In the initial
description of Brugada syndrome, the heart was reported to be
structurally normal, but this concept has been challenged.[5] Subtle structural abnormalities in the right ventricular outflow tract have been reported. Brugada syndrome is genetically determined and has an autosomal dominant pattern of transmission in about 50% of familial cases (see Etiology). The typical patient with Brugada syndrome is young, male, and otherwise healthy, with normal general medical and cardiovascular physical examinations.
Patients with Brugada syndrome are prone to develop ventricular tachyarrhythmias that may lead to syncope, cardiac arrest, or sudden cardiac death.[6, 7, 8] Infrahisian conduction delay and atrial fibrillation may also be manifestations of the syndrome.[9, 10]
About 5% of survivors of cardiac arrest have no clinically identified cardiac abnormality. About half of these cases are thought to be due to Brugada syndrome.[11]
At present, implantation of an automatic implantable cardiac defibrillator (ICD) is the only treatment proven effective in treating ventricular tachycardia and fibrillation and preventing sudden death in patients with Brugada syndrome (see Treatment).
Pathophysiology
Brugada
syndrome is an example of a channelopathy, a disease caused by an
alteration in the transmembrane ion currents that together constitute
the cardiac action potential. Specifically, in 10-30% of cases,
mutations in the SCN5A gene, which encodes the cardiac voltage-gated sodium channel Nav 1.5, have been found. These loss-of-function mutations reduce the sodium current (INa) available during the phases 0 (upstroke) and 1 (early repolarization) of the cardiac action potential.
This decrease in INa is thought to affect the right ventricular endocardium differently from the epicardium. Thus, it underlies both the Brugada ECG pattern and the clinical manifestations of the Brugada syndrome.
The exact mechanisms underlying the ECG alterations and arrhythmogenesis in Brugada syndrome are disputed.[12] The repolarization-defect theory is based on the fact that right ventricular epicardial cells display a more prominent notch in the action potential than endocardial cells. This is thought to be due to an increased contribution of the transient outward current (Ito) to the action potential waveform in that tissue.
A decrease in INa accentuates this difference, causing a voltage gradient during repolarization and the characteristic ST elevations on ECG. Research has provided human evidence for a repolarization gradient in patients with Brugada syndrome using simultaneous endocardial and epicardial unipolar recordings.[13] See the image below.
 Schematics
show the 3 types of action potentials in the right ventricle:
endocardial (End), mid myocardial (M), and epicardial (Epi). A, Normal
situation on V2 ECG generated by transmural voltage gradients during the
depolarization and repolarization phases of the action potential. B-E,
Different alterations of the epicardial action potential that produce
the ECG changes observed in patients with Brugada syndrome. Adapted from
Antzelevitch, 2005. When the usual relative durations of
repolarization are not altered, the T wave remains upright, causing a
saddleback ECG pattern (type 2 or 3). When the alteration in
repolarization is sufficient to cause a reversal of the normal gradient
of repolarization, the T wave inverts, and the coved (type 1) ECG
pattern is seen. In a similar way, a heterogeneous alteration in cardiac
repolarization may predispose to the development of reentrant
arrhythmias, termed phase 2 reentry, that can clinically cause ventricular tachycardia and ventricular fibrillation.[14]
Schematics
show the 3 types of action potentials in the right ventricle:
endocardial (End), mid myocardial (M), and epicardial (Epi). A, Normal
situation on V2 ECG generated by transmural voltage gradients during the
depolarization and repolarization phases of the action potential. B-E,
Different alterations of the epicardial action potential that produce
the ECG changes observed in patients with Brugada syndrome. Adapted from
Antzelevitch, 2005. When the usual relative durations of
repolarization are not altered, the T wave remains upright, causing a
saddleback ECG pattern (type 2 or 3). When the alteration in
repolarization is sufficient to cause a reversal of the normal gradient
of repolarization, the T wave inverts, and the coved (type 1) ECG
pattern is seen. In a similar way, a heterogeneous alteration in cardiac
repolarization may predispose to the development of reentrant
arrhythmias, termed phase 2 reentry, that can clinically cause ventricular tachycardia and ventricular fibrillation.[14]
An alternative hypothesis, the depolarization/conduction disorder model, proposes that the typical Brugada ECG findings can be explained by slow conduction and activation delays in the right ventricle (in particular in the right ventricular outflow tract).[12]
One study used ajmaline provocation to elicit a type 1 Brugada ECG pattern in 91 patients, and found that the repolarization abnormalities were concordant with the depolarization abnormalities and appeared to be secondary to the depolarization changes.[15] Using vectorcardiograms and body surface potential maps, investigators were able to show that depolarization abnormalities and conduction delay mapped to the right ventricle.
This decrease in INa is thought to affect the right ventricular endocardium differently from the epicardium. Thus, it underlies both the Brugada ECG pattern and the clinical manifestations of the Brugada syndrome.
The exact mechanisms underlying the ECG alterations and arrhythmogenesis in Brugada syndrome are disputed.[12] The repolarization-defect theory is based on the fact that right ventricular epicardial cells display a more prominent notch in the action potential than endocardial cells. This is thought to be due to an increased contribution of the transient outward current (Ito) to the action potential waveform in that tissue.
A decrease in INa accentuates this difference, causing a voltage gradient during repolarization and the characteristic ST elevations on ECG. Research has provided human evidence for a repolarization gradient in patients with Brugada syndrome using simultaneous endocardial and epicardial unipolar recordings.[13] See the image below.
 Schematics
show the 3 types of action potentials in the right ventricle:
endocardial (End), mid myocardial (M), and epicardial (Epi). A, Normal
situation on V2 ECG generated by transmural voltage gradients during the
depolarization and repolarization phases of the action potential. B-E,
Different alterations of the epicardial action potential that produce
the ECG changes observed in patients with Brugada syndrome. Adapted from
Antzelevitch, 2005. When the usual relative durations of
repolarization are not altered, the T wave remains upright, causing a
saddleback ECG pattern (type 2 or 3). When the alteration in
repolarization is sufficient to cause a reversal of the normal gradient
of repolarization, the T wave inverts, and the coved (type 1) ECG
pattern is seen. In a similar way, a heterogeneous alteration in cardiac
repolarization may predispose to the development of reentrant
arrhythmias, termed phase 2 reentry, that can clinically cause ventricular tachycardia and ventricular fibrillation.[14]
Schematics
show the 3 types of action potentials in the right ventricle:
endocardial (End), mid myocardial (M), and epicardial (Epi). A, Normal
situation on V2 ECG generated by transmural voltage gradients during the
depolarization and repolarization phases of the action potential. B-E,
Different alterations of the epicardial action potential that produce
the ECG changes observed in patients with Brugada syndrome. Adapted from
Antzelevitch, 2005. When the usual relative durations of
repolarization are not altered, the T wave remains upright, causing a
saddleback ECG pattern (type 2 or 3). When the alteration in
repolarization is sufficient to cause a reversal of the normal gradient
of repolarization, the T wave inverts, and the coved (type 1) ECG
pattern is seen. In a similar way, a heterogeneous alteration in cardiac
repolarization may predispose to the development of reentrant
arrhythmias, termed phase 2 reentry, that can clinically cause ventricular tachycardia and ventricular fibrillation.[14] An alternative hypothesis, the depolarization/conduction disorder model, proposes that the typical Brugada ECG findings can be explained by slow conduction and activation delays in the right ventricle (in particular in the right ventricular outflow tract).[12]
One study used ajmaline provocation to elicit a type 1 Brugada ECG pattern in 91 patients, and found that the repolarization abnormalities were concordant with the depolarization abnormalities and appeared to be secondary to the depolarization changes.[15] Using vectorcardiograms and body surface potential maps, investigators were able to show that depolarization abnormalities and conduction delay mapped to the right ventricle.
Etiology
The prototypical case of Brugada syndrome has been associated with alterations in the SCN5A gene, of which nearly 300 mutations have been described.[16] Mutations
in other genes have been proposed to cause a variant of Brugada
syndrome, including the genes coding for alpha1- and beta2b-subunits of
the L-type calcium channel (CACNA1C and CACNB2), which are thought to
cause a syndrome of precordial ST elevation, sudden death, and short QT
interval.[17]
Mutations in the genes GPD1-L[18] and SCN1B[19] have been identified in a few familial cases. Cases in which a mutation in the SCN5A gene cannot be demonstrated may be due to mutations of these genes, due to other unidentified genes, or located in regions of the coding sequence or promoter region of SCN5A that are not routinely sequenced in lab tests.
Many clinical situations have been reported to unmask or exacerbate the ECG pattern of Brugada syndrome. Examples are a febrile state, hyperkalemia, hypokalemia, hypercalcemia, alcohol or cocaine intoxication, and the use of certain medications, including sodium channel blockers, vagotonic agents, alpha-adrenergic agonists, beta-adrenergic blockers, heterocyclic antidepressants, and a combination of glucose and insulin.[14]
Brugada syndrome is 8-10 times more prevalent in men than in women, although the probability of having a mutated gene does not differ by sex. The penetrance of the mutation therefore appears to be much higher in men than in women.
Brugada syndrome most commonly affects otherwise healthy men aged 30-50 years, but affected patients aged 0-84 years have been reported. The mean age of patients who die suddenly is 41 years.[14]
Mutations in the genes GPD1-L[18] and SCN1B[19] have been identified in a few familial cases. Cases in which a mutation in the SCN5A gene cannot be demonstrated may be due to mutations of these genes, due to other unidentified genes, or located in regions of the coding sequence or promoter region of SCN5A that are not routinely sequenced in lab tests.
Many clinical situations have been reported to unmask or exacerbate the ECG pattern of Brugada syndrome. Examples are a febrile state, hyperkalemia, hypokalemia, hypercalcemia, alcohol or cocaine intoxication, and the use of certain medications, including sodium channel blockers, vagotonic agents, alpha-adrenergic agonists, beta-adrenergic blockers, heterocyclic antidepressants, and a combination of glucose and insulin.[14]
Epidemiology
United States statistics
Because of its recent identification, the prevalence of Brugada syndrome is not well established. In a large university hospital on the West Coast of the United States, the prevalence of a Brugada ECG pattern among unselected, mainly white and Hispanic adults was 2 of 1348 patients (0.14%); in both cases, the ECG patterns were type 2.[20] The prevalence in Asian and other ethnic populations may be higher.International statistics
In parts of Asia (eg, the Philippines, Thailand, Japan), Brugada syndrome seems to be the most common cause of natural death in men younger than 50 years. It is known as Lai Tai (Thailand), Bangungot (Philippines), and Pokkuri (Japan). In Northeast Thailand, the mortality rate from Lai Tai is approximately 30 cases per 100,000 population per year.[21]Race-, sex-, and age-related demographics
Brugada syndrome is most common in people from Asia. The reason for this observation is not yet fully understood but may be due to an Asian-specific sequence in the promoter region of SCN5A.[22]Brugada syndrome is 8-10 times more prevalent in men than in women, although the probability of having a mutated gene does not differ by sex. The penetrance of the mutation therefore appears to be much higher in men than in women.
Brugada syndrome most commonly affects otherwise healthy men aged 30-50 years, but affected patients aged 0-84 years have been reported. The mean age of patients who die suddenly is 41 years.[14]
Prognosis
Brugada syndrome is a cause of polymorphic ventricular tachycardia, which may degenerate into ventricular fibrillation
and cause cardiac arrest. Prolonged hypoxia during cardiac arrest may
leave patients with neurologic sequelae. Implantable
cardioverters-defibrillators (ICDs) are often used to treat patients
with Brugada syndrome, exposing them to complications related to device
implantation and the potential for inappropriate shocks.
During a mean follow-up of 24 months, sudden cardiac death or ventricular fibrillation occurred in 8.2% of patients with Brugada syndrome. A history of syncope, a spontaneously abnormal ECG, and inducibility during programmed electrical stimulation (by one study) significantly increased this risk.[7]
Brugada syndrome may be a significant cause of death, aside from accidents, in men under 40. The true incidence is not known due to reporting biases. Although there is a strong population dependence, an estimated 4% of all sudden deaths and at least 20% of sudden deaths in patients with structurally normal hearts are due to the syndrome. Those with the syndrome have a mean age of sudden death of 41 ±15 years.[23]
During a mean follow-up of 24 months, sudden cardiac death or ventricular fibrillation occurred in 8.2% of patients with Brugada syndrome. A history of syncope, a spontaneously abnormal ECG, and inducibility during programmed electrical stimulation (by one study) significantly increased this risk.[7]
Brugada syndrome may be a significant cause of death, aside from accidents, in men under 40. The true incidence is not known due to reporting biases. Although there is a strong population dependence, an estimated 4% of all sudden deaths and at least 20% of sudden deaths in patients with structurally normal hearts are due to the syndrome. Those with the syndrome have a mean age of sudden death of 41 ±15 years.[23]
Patient Education
Educating the patient and his or her family members and coworkers about basic cardiopulmonary resuscitation (CPR) is important. Genetic counseling is reasonable if desired by the patient and familyHistory
Syncope and cardiac arrest are the most common clinical manifestations leading to the diagnosis of Brugada syndrome. Nightmares or thrashing at night may occur. However, some patients remain asymptomatic, and the diagnosis of Brugada syndrome is suggested by a routine ECG showing ST-segment elevation in leads V1 through V3.A family history of sudden cardiac death is common, though not universal, as the syndrome can occur sporadically. In about 20% of patients, atrial fibrillation is an associated arrhythmia.[2]
The context of the cardiac event is important. In many cases, cardiac arrest occurs during sleep or rest. Cases occurring during physical activity are rare. Fever is often reported to trigger or exacerbate the clinical manifestations of Brugada syndrome.
A 2012 study suggests that the quality of symptoms prior to syncope can predict a benign or malignant cause in patients with Brugada syndrome. Specifically, the lack of a prodrome was more common in patients with ventricular fibrillation documented as the cause of syncope.[3]
Physical Examination
The physical examination is usually normal in patients with Brugada syndrome. Nevertheless, physical examination is required to rule out other possible cardiac causes of syncope or cardiac arrest in an otherwise healthy patient (eg, heart murmurs from hypertrophic cardiomyopathy or from a valvular or septal defectDiagnostic Considerations
The differential diagnosis of cardiac arrest in an otherwise presumably healthy subject is varied, but it includes such entities as acute cardiac ischemia due to atherosclerosis or coronary anomaly, hypertrophic cardiomyopathy, catecholaminergic polymorphic ventricular tachycardia, long QT syndrome, and arrhythmogenic right ventricular cardiomyopathy (ARVC). Many of these entities can be differentiated on the basis of history and physical examination. Occasionally, however, there is overlap that requires special consideration.The differential diagnosis of right precordial ST-segment elevation is as follows[14, 24] :
- Atypical right bundle-branch block
- Left ventricular hypertrophy
- Early repolarization
- Acute pericarditis
- Acute myocardial ischemia or infarction
- Prinzmetal angina
- Pulmonary embolism
- Dissecting aortic aneurysm
- Mediastinal tumor or hemopericardium compressing the right ventricular outflow tract
- Arrhythmogenic right ventricular dysplasia and/or cardiomyopathy
- Various abnormalities of the central and autonomic nervous systems
- Overdose of a heterocyclic antidepressant
- Cocaine intoxication
- Duchenne muscular dystrophy
- Friedreich ataxia
- Thiamine deficiency Hypercalcemia
- Hyperkalemia
- Hypothermia
- Pectus excavatum
- Effects of athletic training
Differential Diagnoses
Approach Considerations
Many
patients with Brugada syndrome are young and otherwise healthy and may
present with syncope. Patients with syncope should not be assumed to
have a benign condition, and a 12-lead ECG should be performed.
A drug challenge with a sodium channel blocker should be considered in patients with syncope in whom no obvious cause is found. An experienced physician should interpret the ECGs, and an electrophysiologist should review them if possible.
Further testing may be indicated to exclude other diagnostic possibilities.
A drug challenge with a sodium channel blocker should be considered in patients with syncope in whom no obvious cause is found. An experienced physician should interpret the ECGs, and an electrophysiologist should review them if possible.
Further testing may be indicated to exclude other diagnostic possibilities.
Electrocardiography
Three ECG patterns have been described in Brugada syndrome[24] (see
the image and table below). Placing the right precordial leads in the
second intercostal space has been proposed to add sensitivity to the ECG
diagnosis of Brugada syndrome.[25] Exercise stress testing may suppress ECG changes and arrhythmias.
 Three
types of ST-segment elevation in Brugada syndrome, as shown in the
precordial leads on ECG in the same patient at different times. Left
panel shows a type 1 ECG pattern with pronounced elevation of the J
point (arrow), a coved-type ST segment, and an inverted T wave in V1 and
V2. The middle panel illustrates a type 2 pattern with a saddleback
ST-segment elevated by >1 mm. The right panel shows a type 3 pattern
in which the ST segment is elevated < 1 mm. According to a consensus
report (Antzelevitch, 2005), the type 1 ECG pattern is diagnostic of
Brugada syndrome. Modified from Wilde, 2002. Table. ECG Patterns in Brugada Syndrome (Open Table in a new window)
Three
types of ST-segment elevation in Brugada syndrome, as shown in the
precordial leads on ECG in the same patient at different times. Left
panel shows a type 1 ECG pattern with pronounced elevation of the J
point (arrow), a coved-type ST segment, and an inverted T wave in V1 and
V2. The middle panel illustrates a type 2 pattern with a saddleback
ST-segment elevated by >1 mm. The right panel shows a type 3 pattern
in which the ST segment is elevated < 1 mm. According to a consensus
report (Antzelevitch, 2005), the type 1 ECG pattern is diagnostic of
Brugada syndrome. Modified from Wilde, 2002. Table. ECG Patterns in Brugada Syndrome (Open Table in a new window)
Recently, the QRS duration on 12-lead ECG has been suggested as a risk marker for vulnerability to dangerous arrhythmias.[26, 27] Inferolateral repolarization abnormalities have also been proposed to be a marker of risk.[28, 29]
Asymptomatic patients with a type 1 ECG pattern on routine ECG represent a difficult case. According to the latest consensus guidelines, a clinical electrophysiologist should evaluate patients in this situation.[14] Patients should be risk-stratified using the techniques described below, and a decision on implantable cardioverter-defibrillator (ICD) implantation should be made accordingly.
In patients with a normal baseline ECG, the results are positive when the drug generates a J wave with an absolute amplitude of 2 mm or more in leads V1, V2, and/or V3 with or without an RBBB. Administration of the drug should be stopped when the result is positive, when ventricular arrhythmia occurs, or when QRS widening of greater than 30% is observed.
Isoproterenol and sodium lactate may be effective as antidotes if the sodium channel blocker induces an arrhythmia, and the isoproterenol response may also have diagnostic use.
This drug test should not be performed in patients with a type 1 ECG pattern (see Table above) because it adds no new information.
In patients with the type 2 or 3 patterns, the drug challenge is recommended to clarify the diagnosis.[14] The sensitivity and specificity of drug challenge testing is not yet confirmed. A 2012 study examining patients with type 2 or 3 patterns showed that a positive drug challenge in symptomatic patients was associated with adverse events. However, asymptomatic patients with a similar result had a low event rate. This study suggests that a drug challenge may aid in risk stratification for symptomatic patients with a nondiagnostic ECG, but may not be justified in asymptomatic patients.[30]
 Three
types of ST-segment elevation in Brugada syndrome, as shown in the
precordial leads on ECG in the same patient at different times. Left
panel shows a type 1 ECG pattern with pronounced elevation of the J
point (arrow), a coved-type ST segment, and an inverted T wave in V1 and
V2. The middle panel illustrates a type 2 pattern with a saddleback
ST-segment elevated by >1 mm. The right panel shows a type 3 pattern
in which the ST segment is elevated < 1 mm. According to a consensus
report (Antzelevitch, 2005), the type 1 ECG pattern is diagnostic of
Brugada syndrome. Modified from Wilde, 2002. Table. ECG Patterns in Brugada Syndrome (Open Table in a new window)
Three
types of ST-segment elevation in Brugada syndrome, as shown in the
precordial leads on ECG in the same patient at different times. Left
panel shows a type 1 ECG pattern with pronounced elevation of the J
point (arrow), a coved-type ST segment, and an inverted T wave in V1 and
V2. The middle panel illustrates a type 2 pattern with a saddleback
ST-segment elevated by >1 mm. The right panel shows a type 3 pattern
in which the ST segment is elevated < 1 mm. According to a consensus
report (Antzelevitch, 2005), the type 1 ECG pattern is diagnostic of
Brugada syndrome. Modified from Wilde, 2002. Table. ECG Patterns in Brugada Syndrome (Open Table in a new window)| Characteristic | Type 1 | Type 2 | Type 3 |
| J wave amplitude | ≥2 mm | ≥2 mm | ≥2 mm |
| T wave | Negative | Positive or biphasic | Positive |
| ST-T configuration | Cover-type | Saddleback | Saddleback |
| ST segment, terminal portion | Gradually descending | Elevated by ≥1 mm | Elevated by < 1 mm |
Asymptomatic patients with a type 1 ECG pattern on routine ECG represent a difficult case. According to the latest consensus guidelines, a clinical electrophysiologist should evaluate patients in this situation.[14] Patients should be risk-stratified using the techniques described below, and a decision on implantable cardioverter-defibrillator (ICD) implantation should be made accordingly.
Signal-averaged ECG
Arrhythmogenic right ventricular cardiomyopathy (ARVC) and Brugada syndrome may be difficult to differentiate in some cases. Late potentials on signal-averaged ECG may reveal the fibrofatty degeneration of the right ventricle seen in ARVC.Challenge with sodium channel blockers
In some patients, the intravenous administration of drugs that block sodium channels may unmask or modify the ECG pattern, aiding in diagnosis and/or risk stratification in some individuals. Infuse flecainide 2 mg/kg (maximum 150 mg) over 10 minutes, procainamide 10 mg/kg over 10 minutes, ajmaline 1 mg/kg over 5 minutes, or pilsicainide 1 mg/kg over 10 minutes. This challenge should be performed with continuous cardiac monitoring and in a setting equipped for resuscitation.In patients with a normal baseline ECG, the results are positive when the drug generates a J wave with an absolute amplitude of 2 mm or more in leads V1, V2, and/or V3 with or without an RBBB. Administration of the drug should be stopped when the result is positive, when ventricular arrhythmia occurs, or when QRS widening of greater than 30% is observed.
Isoproterenol and sodium lactate may be effective as antidotes if the sodium channel blocker induces an arrhythmia, and the isoproterenol response may also have diagnostic use.
This drug test should not be performed in patients with a type 1 ECG pattern (see Table above) because it adds no new information.
In patients with the type 2 or 3 patterns, the drug challenge is recommended to clarify the diagnosis.[14] The sensitivity and specificity of drug challenge testing is not yet confirmed. A 2012 study examining patients with type 2 or 3 patterns showed that a positive drug challenge in symptomatic patients was associated with adverse events. However, asymptomatic patients with a similar result had a low event rate. This study suggests that a drug challenge may aid in risk stratification for symptomatic patients with a nondiagnostic ECG, but may not be justified in asymptomatic patients.[30]
Electrophysiologic Study
Some
investigators use an electrophysiologic study (EPS) to determine the
inducibility of arrhythmias, in an effort to risk-stratify patients with
Brugada syndrome. However, the predictive value of this approach is
debated. In 2001, Brugada showed that inducibility may be a good
predictor of outcome.[31] However, in 2002, Priori reported a poor predictive value of invasive testing.[32] A subsequent study by Gehi concluded that EPS was not of use in guiding the management of patients with Brugada syndrome.[33]
More recently, investigators independently examining a large series of patients from Europe and Japan have failed to find any predictive value for EPS. In the large registry of Brugada syndrome patients from Europe, only symptoms and a spontaneous type 1 Brugada ECG pattern, but not EPS, were predictive of arrhythmic events.[34] . In the smaller Japanese registry, only family history of sudden cardiac death at younger than 45 years and inferolateral early repolarization pattern on ECG predicted cardiac events.[29]
A study by Priori et al enrolled 308 patients with no history of cardiac arrest and a spontaneous or drug-induced type I ECG pattern.[35] Seventy eight of the patients had an ICD implanted prophylactically. EPS with a consistent stimulation protocol was performed on all patients; at a mean follow-up of 34 months, no differences were found in the incidence of appropriate ICD shocks or cardiac arrest between patients who were inducible and patients who were noninducible. Significant predictors of arrhythmia in this study included syncope and a spontaneous type I ECG pattern, a ventricular effective refractory period of less than 200 ms on EPS, and a fragmented QRS in the anterior precordial ECG leads.
Investigators from the United Kingdom examined a group of probands who suffered sudden arrhythmic death believed to be due to Brugada syndrome.[36] A retrospective review of risk factors determined that these patients would not have been considered high risk, calling into question the sensitivity of current risk factors (eg, symptoms, type I ECG pattern). However, few ECGs were available for examination in the probands with sudden death .
More recently, investigators independently examining a large series of patients from Europe and Japan have failed to find any predictive value for EPS. In the large registry of Brugada syndrome patients from Europe, only symptoms and a spontaneous type 1 Brugada ECG pattern, but not EPS, were predictive of arrhythmic events.[34] . In the smaller Japanese registry, only family history of sudden cardiac death at younger than 45 years and inferolateral early repolarization pattern on ECG predicted cardiac events.[29]
A study by Priori et al enrolled 308 patients with no history of cardiac arrest and a spontaneous or drug-induced type I ECG pattern.[35] Seventy eight of the patients had an ICD implanted prophylactically. EPS with a consistent stimulation protocol was performed on all patients; at a mean follow-up of 34 months, no differences were found in the incidence of appropriate ICD shocks or cardiac arrest between patients who were inducible and patients who were noninducible. Significant predictors of arrhythmia in this study included syncope and a spontaneous type I ECG pattern, a ventricular effective refractory period of less than 200 ms on EPS, and a fragmented QRS in the anterior precordial ECG leads.
Investigators from the United Kingdom examined a group of probands who suffered sudden arrhythmic death believed to be due to Brugada syndrome.[36] A retrospective review of risk factors determined that these patients would not have been considered high risk, calling into question the sensitivity of current risk factors (eg, symptoms, type I ECG pattern). However, few ECGs were available for examination in the probands with sudden death .
Potassium and Calcium Levels
Check serum potassium and calcium levels in patients presenting with ST-segment elevation in the right precordial leads.
Both hypercalcemia and hyperkalemia may generate an ECG pattern similar to that of Brugada syndrome.
Both hypercalcemia and hyperkalemia may generate an ECG pattern similar to that of Brugada syndrome.
Creatine Kinase-MB and Troponin
Laboratory
markers, such as creatine kinase-MB (CK-MB) and troponin, should be
checked in patients who have symptoms compatible with an acute coronary
syndrome. Elevations indicate cardiac injury.
Genetic Testing
Patients with high likelihood of Brugada syndrome may be genetically tested for a mutation in SCN5A, which codes for the alpha subunit Nav
1.5 of the cardiac sodium channel. The results of this test support the
clinical diagnosis and are important for the early identification of
family members at potential risk. However, the yield of genetic testing
remains relatively low at this time, with mutations in SCN5A found in only 11-28% of index cases.[16]
Echocardiography and/or MRI
Echocardiography
and/or MRI should be performed, mainly to exclude arrhythmogenic right
ventricular cardiomyopathy. However, these studies are also used to
assess for other potential causes of arrhythmias, such as hypertrophic
cardiomyopathy, unsuspected myocardial injury, myocarditis, or aberrant
coronary origins.
Approach Considerations
At
present, implantation of an automatic implantable cardiac defibrillator
(ICD) is the only treatment proven effective in treating ventricular
tachycardia and fibrillation and preventing sudden death in patients
with Brugada syndrome. No pharmacologic therapy has been proven to
reduce the occurrence of ventricular arrhythmias or sudden death.
Indications for ICD implantation were published in the report of the Second Consensus Conference on Brugada syndrome.[23] For patients at the 2 extremes of risk stratification, the decision to implant or not to implant an ICD is relatively straightforward.
Patients with Brugada syndrome and a history of cardiac arrest must be treated with an ICD. In contrast, asymptomatic patients with no family history of sudden cardiac death can be managed conservatively with close follow-up, and ICD implantation is not recommended. Patients with intermediate clinical characteristics present the greatest challenge. For details about risk stratification and indications for ICD implantation, readers are referred to the Second Consensus Conference report.[23]
Indications for ICD implantation were published in the report of the Second Consensus Conference on Brugada syndrome.[23] For patients at the 2 extremes of risk stratification, the decision to implant or not to implant an ICD is relatively straightforward.
Patients with Brugada syndrome and a history of cardiac arrest must be treated with an ICD. In contrast, asymptomatic patients with no family history of sudden cardiac death can be managed conservatively with close follow-up, and ICD implantation is not recommended. Patients with intermediate clinical characteristics present the greatest challenge. For details about risk stratification and indications for ICD implantation, readers are referred to the Second Consensus Conference report.[23]
Activity Restriction
Because
regular physical activity may increase vagal tone, sport may eventually
enhance the propensity of athletes with Brugada syndrome to have
ventricular fibrillation and sudden cardiac death at rest or during
recovery after exercise. Therefore, Pelliccia et al recommend that
patients with a definite diagnosis of Brugada syndrome should be
restricted from competitive sports.[37]
However, no direct evidence supports this recommendation. It remains unclear whether asymptomatic carriers of SCN5A mutations should also be restricted from participation in sports.
However, no direct evidence supports this recommendation. It remains unclear whether asymptomatic carriers of SCN5A mutations should also be restricted from participation in sports.
Management of Concomitant Syncope or Cardiac Arrest
Patients
with syncope or cardiac arrest and suspected or diagnosed Brugada
syndrome must be hospitalized. Continuous cardiac monitoring is
necessary until definitive treatment (ie, ICD placement) can be
provided.
Deterrence/Prevention of Complications
When indicated, use of an ICD may prevent sudden cardiac death.[14] The
patient's relatives and coworkers should be educated about Brugada
syndrome and the basics of cardiopulmonary resuscitation (CPR). Genetic
counseling is indicated if desired by the patient and his or her family.
Consultations
A
board-certified cardiologist who specializes in cardiac arrhythmic
disorders (ie, a clinical electrophysiologist) should evaluate patients
with suspected Brugada syndrome. Consultation with a genetic counselor
is indicated for genetic screening and counseling of patients and their
relatives.
Long-Term Monitoring
A
board-certified electrophysiologist should closely follow patients with
Brugada syndrome. Taking a careful history is important, as not all
syncope is necessarily arrhythmic in Brugada syndrome. For example, a
clear prodrome suggesting vasovagal syncope does not suggest an adverse
prognosis in an otherwise asymptomatic patient with a Brugada ECG
pattern.
Medication Summary
Theoretically,
drugs that counteract the ionic current imbalance in Brugada syndrome
could be used to treat it. For example, quinidine, which blocks the
calcium-independent transient outward potassium current (Ito), has been
shown to normalize the ECG pattern in patients with Brugada syndrome.[4] However, quinidine also blocks sodium (Na) currents, which could have contrary effects.
Tedisamil, a potent Ito blocker without strong Na channel effects, may be more effective than quinidine.[38] Isoproterenol, which boosts the L-type calcium current, can also counteract the ionic current imbalance.[38]
Thus far, no drug therapy for Brugada syndrome is recommended because clinical trials have failed to convincingly prove effectiveness.[23, 39, 40, 41] A number of medications can unmask the Brugada pattern on ECG and potentially exacerbate the clinical manifestations of Brugada syndrome. The Web site BrugadaDrugs.org has been established to educate patients and professionals about these potentially dangerous medications.[42]
Quinidine
maintains a normal heart rhythm following cardioversion of atrial
fibrillation or flutter. It depresses myocardial excitability and
conduction velocity. Prior to administration, control the ventricular
rate and CHF (if present) with digoxin or calcium channel blockers.
Tedisamil, a potent Ito blocker without strong Na channel effects, may be more effective than quinidine.[38] Isoproterenol, which boosts the L-type calcium current, can also counteract the ionic current imbalance.[38]
Thus far, no drug therapy for Brugada syndrome is recommended because clinical trials have failed to convincingly prove effectiveness.[23, 39, 40, 41] A number of medications can unmask the Brugada pattern on ECG and potentially exacerbate the clinical manifestations of Brugada syndrome. The Web site BrugadaDrugs.org has been established to educate patients and professionals about these potentially dangerous medications.[42]
Antiarrhythmic Drugs
Class Summary
There is general consensus that certain drugs can be potentially antiarrhythmic in Brugada syndrome patients. However, there are no randomized clinical studies in Brugada syndrome patients. As such, only in the setting of long-term therapeutic treatment in an experienced medical center can practitioners consider the use of antiarrhythmics for high-risk patients. Currently, quinidine seems to be the treatment of choice for long-term therapy.Quinidine
___________________________
End of Medscape article.
End of Medscape article.
Plumb "I Want You Here"

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.